 The Chemistry of Epoxides The Chemistry of Epoxides
 Reactions of Epoxides Reactions of Epoxides
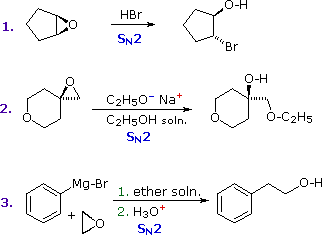
 Epoxides (oxiranes) are three-membered cyclic ethers that are easily prepared from alkenes by reaction with peracids. Because of the large angle strain in this small ring, epoxides undergo acid and base-catalyzed C–O bond cleavage more easily than do larger ring ethers. Epoxides (oxiranes) are three-membered cyclic ethers that are easily prepared from alkenes by reaction with peracids. Because of the large angle strain in this small ring, epoxides undergo acid and base-catalyzed C–O bond cleavage more easily than do larger ring ethers.
 Among the following examples, the first is unexceptional except for the fact that it occurs under milder conditions and more rapidly than other ether cleavages. The second and third examples clearly show the exceptional reactivity of epoxides, since unstrained ethers present in the same reactant or as solvent do not react. Among the following examples, the first is unexceptional except for the fact that it occurs under milder conditions and more rapidly than other ether cleavages. The second and third examples clearly show the exceptional reactivity of epoxides, since unstrained ethers present in the same reactant or as solvent do not react.
 The aqueous acid used to work up the third reaction, following the Grignard reagent cleavage of the ethylene oxide, simply neutralizes the magnesium salt of the alcohol product. The aqueous acid used to work up the third reaction, following the Grignard reagent cleavage of the ethylene oxide, simply neutralizes the magnesium salt of the alcohol product.
|